Understanding how Gilead Sciences may respond to the controversy by analyzing its patent portfolio
by Leo Tsou, Manager at Wispro Technology Consulting Corporation
- Remdesivir, which was developed by the American pharmaceutical company Gilead Sciences, Inc., has shown potential in treating the first patient in America to suffer from the novel coronavirus infection (Covid-19).
- On February 6, the company began clinical trials of the drug in China at frontline hospitals in Wuhan, even offering to provide free Remdesivir in these clinical trials in order to help Wuhan defeat the outbreak.
- At around the same time, however, the Wuhan Institute of Virology—which is part of the China Academy of Sciences—announced that it had already applied for a patent on Gilead Sciences’ Remdesivir for the purpose of treating the infection.
- How will Gilead Sciences respond? This article will focus on an analysis of Gilead Sciences’ Remdesiver-related patents in order to uncover some of the company’s possible responses to this situation.
What happened?
On February 4, the Wuhan Institute of Virology announced that it had applied for a Chinese patent on Gilead’s Remdesivir for novel coronavirus treatment in order to protect the national interests of China. The Chinese patent application was filed on January 21, and it will be filed internationally through the Patent Cooperation Treaty (PCT).
The Institute also stated:
If relevant foreign companies intend to contribute to the prevention and control of China’s epidemic, we both agree that if the state needs it, we will not require the implementation of the rights claimed in the patent for the time being, and we hope to work with foreign pharmaceutical companies to minimize the epidemic.
According to this statement, the Institute’s intention in applying for coronavirus patents is to keep the original patent from having its legal rights practiced. In fact, the goal may just be to establish a cross-licensing agreement with Gilead Sciences, so that neither party will practice its legal rights against the other.
What’s in Gilead Sciences’ portfolio?
Some Chinese executives believe that this move by the Institute may enable China to avoid having to pay steep licensing fees for using the patented drug.
Yet, it should be noted that Gilead Sciences is one of the top 10 pharmaceutical companies in the world. Will it simply sit back and accept this situation?
Based on our analysis utilizing Patentcould’s Due Diligence, Gilead Sciences has already filed 133 coronavirus patents related to Remdesivir in 43 countries and patent offices around the world since 2011.
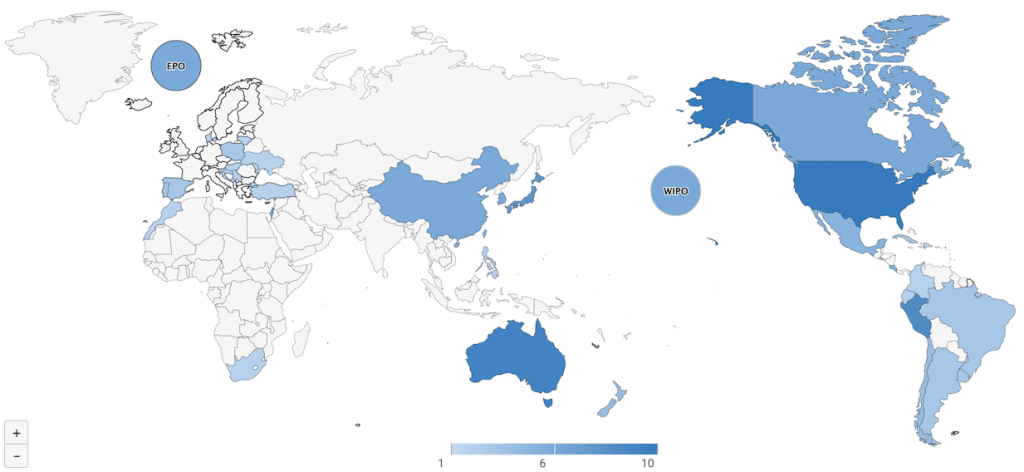
Download the complete list of patents to perform your own analysis
Gilead Sciences’ Remdesivir portfolio comprises 4 major patent families:
- Family 1 and Family 2 both cover the structure of Remdesivir-related compounds
- In addition, Family 2 also covers the manufacturing method of Remdesivir
- Family 3 covers the use for the treatment of Coronaviridae infection
What’s more, Gilead Sciences has also applied for patents related to the uses for treatment of:
- Paramyxoviridae infection (in Family 1)
- Filoviridae infection and Nipah Virus infection (in Family 2)
- Arenaviridae infection (in Family 3)
- Feline Coronavirus infection (in Family 4)
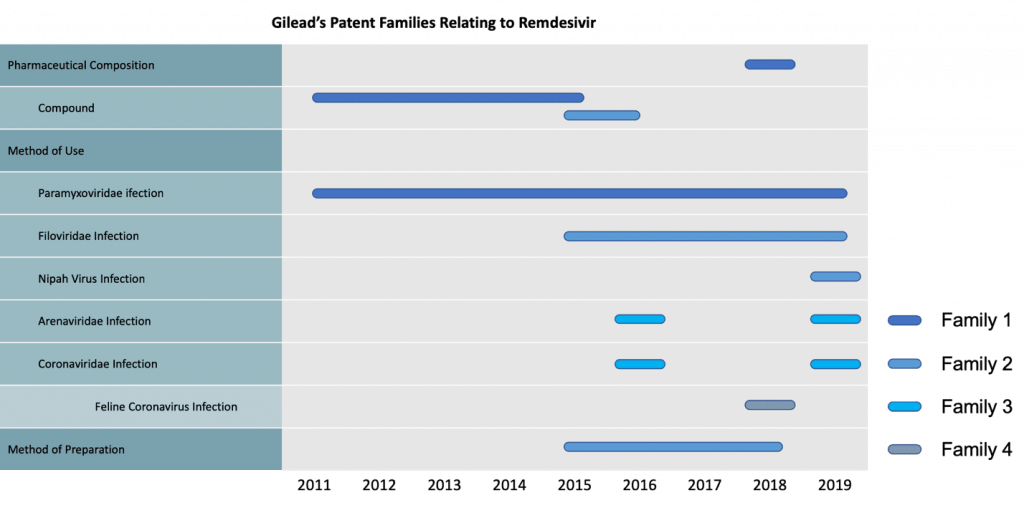
Download the complete list of patents to perform your own analysis
The coronavirus patent filed by the Wuhan Institute of Virology was applied for on January 21, 2020. Yet, it might not be so easy to prove novelty and non-obviousness because of the many prior art references that have already been filed by Gilead Sciences.
Even if the patent can prove its novelty and non-obviousness, it might take about three to five more years for it to be granted in countries outside of China via the PCT application. At that time, it is hard to determine if the novel coronavirus will still be an issue or not.
In fact, Gilead Sciences already has granted coronavirus patents in several countries, including the United States, China, Europe, Japan, and Korea. The patent rights related to the compound structure and the manufacturing process of Remdesivir may extend from 2031 to 2036.
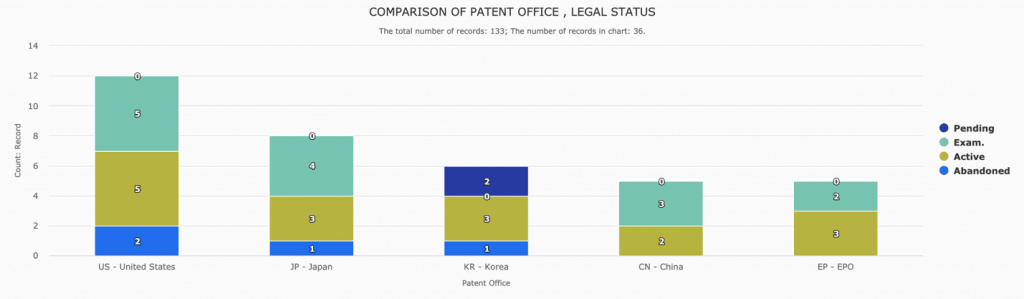
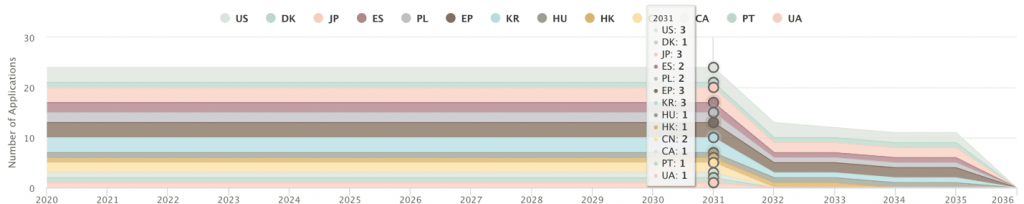
Download the complete list of patents to perform your own analysis
Responding to this issue, Daniel O’Day, the CEO of Gilead Sciences, has stated:
We’re not going to get involved in a patent dispute. We’re going to find ways to help patients, and, of course, we’re going to protect our intellectual property as a separate part of the process, but patients come first.
This statement expressed the sentiment that helping patients in Wuhan is the company’s current first priority; however, the company may still enforce its legal rights, if necessary, in the future.
For the company, business is business, but just what benefits might the US pharmaceuticals gain by providing Wuhan with free test drugs?
Coronavirus patents: a second chance for Remdesivir
As we can see in Gilead Sciences’ patent filing history, the company actually began to develop Remdesivir in 2011. In 2018, when the Ebola outbreak occurred in Africa, the company planned to develop Remdesivir as a drug for treating the Ebola virus infection.
Unfortunately, clinical trials showed that the efficacy of Remdesivir did not match that of two other comparative drugs. This was definitely a blow for Gilead Sciences, since the nine years of investment in R&D must have cost the company a lot.
But now, the novel coronavirus outbreak has given the failed Ebola drug a second chance. Clinical trials of Gilead Sciences’ Remdesivir have now begun in Wuhan, and they are planned to involve more than 700 patients with mild, moderate, and severe Covid-19 infections.
To solve the urgent problem caused by the novel coronavirus, it is highly possible that all regulatory agencies and hospitals will provide their full support to accelerate the approval of Remdesivir. If the trial recruitment goes well and Remdesivir turns out to be an effective treatment against Covid-19, it might not take long for it to get the approval.
If this is the case, Remdesivir will be the only approved product in treating the Covid-19 infection, and global demand for Remdesivir would likely be huge. From this point of view, offering free drugs to patients in Wuhan may end up benefiting not only China and the rest of the world, but Gilead Sciences in particular.

