Using patent data analysis to uncover universal flu vaccine development companies.
– by Leo Tsou, Manager of Wispro Technology Consulting Corporation
In early February, the president of the United States, Donald Trump once again announced a 7% cut to the budget, which works out at around 30 billion from the National Institutes of Health (NIH) while increasing 2 billion in funding for the development of universal vaccines.
Data from the Centers for Disease Control and Prevention (CDC) has shown that from October 2019 until mid-February 2020, nearly 500,000 citizens in the United States were hospitalized with the flu, of which 41,000 died, this severity far exceeds the current outbreak of the novel coronavirus.
This alarming data posed the following questions:
What is a universal flu vaccine? Why is it needed? Which major companies are currently developing universal influenza vaccines?
This article leverages InQuartik’s Patentcloud, a patent intelligence platform for patent data analysis. Additionally, this article provides a detailed introduction to the current development status of universal influenza vaccines.
Why Do We Need a Universal Flu Vaccine?
The surface antigen of influenza virus is highly variable. This high variability may be caused by the viral antigen’s high mutation rate, which is called an antigenic drift or homologous recombination, and they occur when the virus cross-infects between different hosts, such as birds, pigs, etc. The varied virus could become a brand new virus to our immune systems; This is why it is possible to catch the flu next year even if you’ve already had it this year.
Regarding the current seasonal flu vaccine strategy, the WHO predicts possible outbreak viral strains for the following year based on the viral strains of seasonal flu from the previous year, and proposes three to four of the most likely strains to the vaccine companies. These companies will then proceed to produce attenuated virus vaccines to protect against these predicted viral strains.
Since there are too many uncontrollable factors in nature, it is almost impossible to predict the virus accurately. According to statistics from the CDC, the flu vaccination only reduces the risk of flu illness by between 40% and 60%, which is not an ideal outcome.
In addition to the seasonal flu, virus mutations may also cause influenza and various other viruses that would not usually infect humans, e.g., swine flu. These other viruses could eventually gain the ability to infect humans, and humans are likely to have no resistance to such illnesses.
Once a pandemic flu outbreak occurs, it is often difficult to contain; for example, the Spanish flu that happened in 1918 killed between 50-100 million people worldwide (the world’s population at the time was roughly 1.7 billion.)
For the reasons above, many scholars and biotechnology companies have tried to develop a universal flu vaccine since the late 1990s, many of them failed. But now, there are some companies, most of which are different from those original companies, have discovered better solutions, and are now one step closer to success.
We utilized Patentcloud’s Patent Vault database and patent data to analyze eight of the universal influenza vaccine development companies with the most significant potential, including BiondVax, Osivax, FluGen, Vivaldi Biosciences, Blue Water Vaccines, Emergex Vaccines, Vaccitech, and Distributed Bio.
This article will introduce these companies through their development progress, technology, and patent deployment to uncover the current situation of the universal influenza vaccine industry.
The solutions for universal influenza vaccine development can be divided into recombinant subunit vaccines, live attenuated virus, and virus-like particles (VLPs.)
The recombinant subunit vaccine refers to the use of recombinant proteins containing complete or partial viral antigens. Since its main component is a purified protein that does not contain viral nucleic acids, it does not run the risk of causing viral infections and is relatively safe for humans. Companies developing recombinant subunit vaccines include BiondVax, Osivax, Emergex Vaccines, and Distributed Bio.
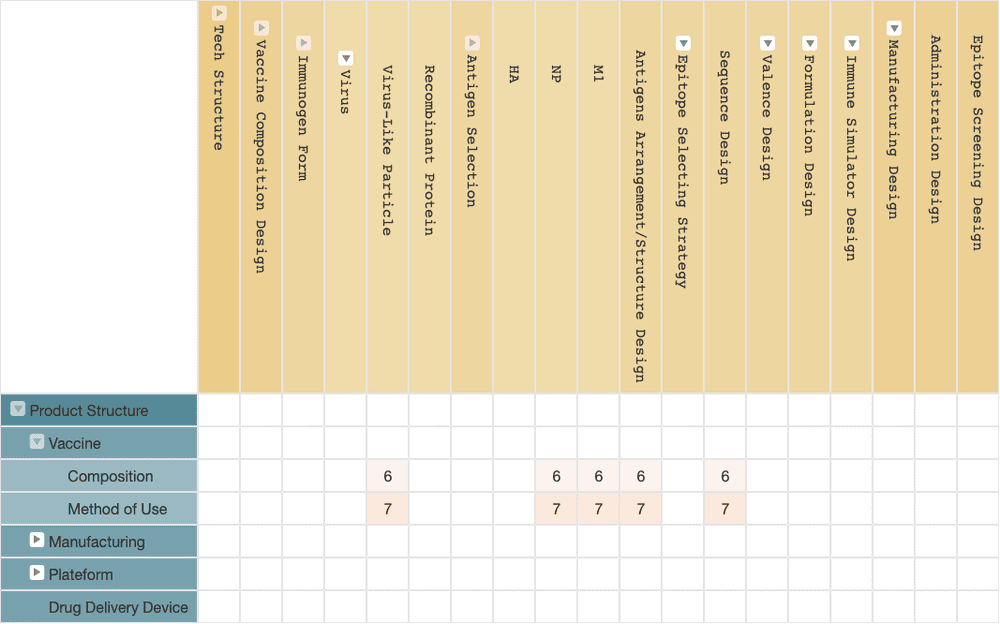
BiondVax
Due to the high variability of influenza viruses, it is difficult to find a conserved region that is not prone to mutate between different influenza strains on a single viral antigen and trigger a strong enough immune response at the same time.
To overcome this issue, BiondVax adopts a more complicated design. According to the information from their patent applications, the solution of BiondVax defines a total of 9 conserved regions in 3 viral antigens: hemagglutinin (HA), nucleoprotein (NP), and matrix protein 1 (M1).
Then they select multiple epitopes from these nine conserved regions, and arrange the epitopes into a long string of recombinant proteins in multiple repetition, attempting to use as many conserved epitopes of viral antigens as possible to develop an immune affection that is sufficient to cover all types of influenza virus strains.
BiondVax’s M-001 is the earliest universal flu vaccine that has entered the phase III clinical trial. The trial started in 2018 and is expected to finish this year. The initial clinical trials began in 2009, and the relevant patent applications were filed in 2008 and have continued to be applied ever since. The patent applications mainly focus on vaccine compositions.
(Source: Wispro Technology Consulting Corporation, 2020)
Osivax
Unlike BiondVax, Osivax‘s vaccine design uses a single viral antigen. The key technology of Osivax is called OligoDOM, and it is applied to enhance the vaccine’s immune response.
OligoDOM technology is the aggregation of 7 viral NP antigens into a nanoparticle. Each NP antigen has additional “tails” that are positively charged short peptides, which allows the antigen nanoparticles to enter the dendritic cells more efficiently, thus achieving the simulation of the effect of CD8 + T cell immune response.
The nanoparticle design allows Osivax’s universal flu vaccine, OVX836, to be administered through a nasal spray, like an attenuated viral vaccine, even though it is a recombinant protein vaccine. The injectable formulation of OVX836 is undergoing its phase II clinical trial, while its nasal spray formulation is undergoing its phase I clinical trial simultaneously.
Osivax was a spin-off subsidiary of the French pharmaceutical company IMAXIO back in 2017. Osivax’s current patent portfolios are all derived from IMAXIO, and the two companies jointly hold the patents. Their patent portfolios are mainly of vaccine compositions using their OligoDOM technology and its methods of use.
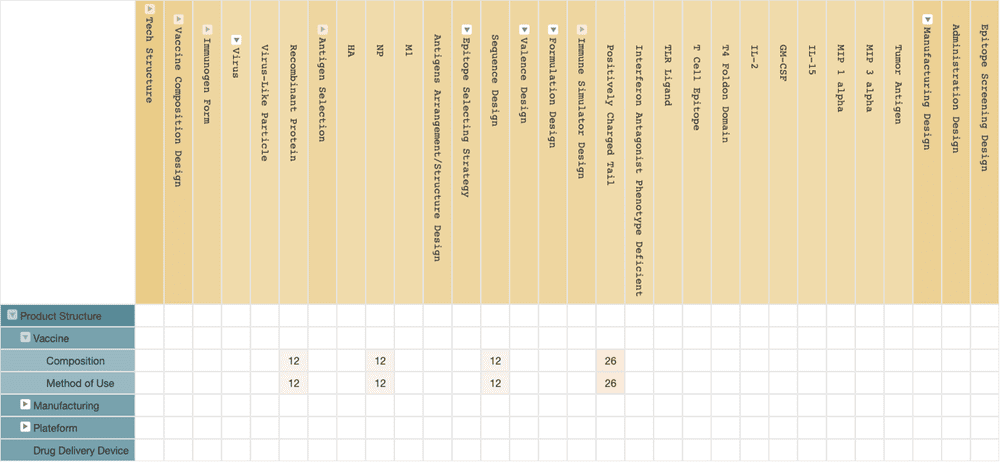
(Source: Wispro Technology Consulting Corporation, 2020)
Emergex Vaccines
Emergex Vaccines‘s universal flu vaccine is not yet in its clinical trial stage. They have a core technology platform, MHC class I CD8+ T-cell ligandome library, comprising a reservoir of various viral peptide fragments that has CD8+ T cell-stimulating properties. It is being applied to the development of universal vaccines for influenza viruses, yellow fever viruses such as dengue virus and Zika virus, and filamentous viruses such as Ebola.
Regarding the patent protection for their universal influenza virus vaccine development, Emergex Vaccines has applied for the compositions and its use of an influenza vaccine containing both CD8+ T-cell epitope and B-cell epitope from a viral antigen. Besides, Emergex Vaccines also filed patents covering the company’s quantum-size nanocluster delivery system. However, as of the publishing date of this article, there is no granted patent owned by Emergex Vaccines.
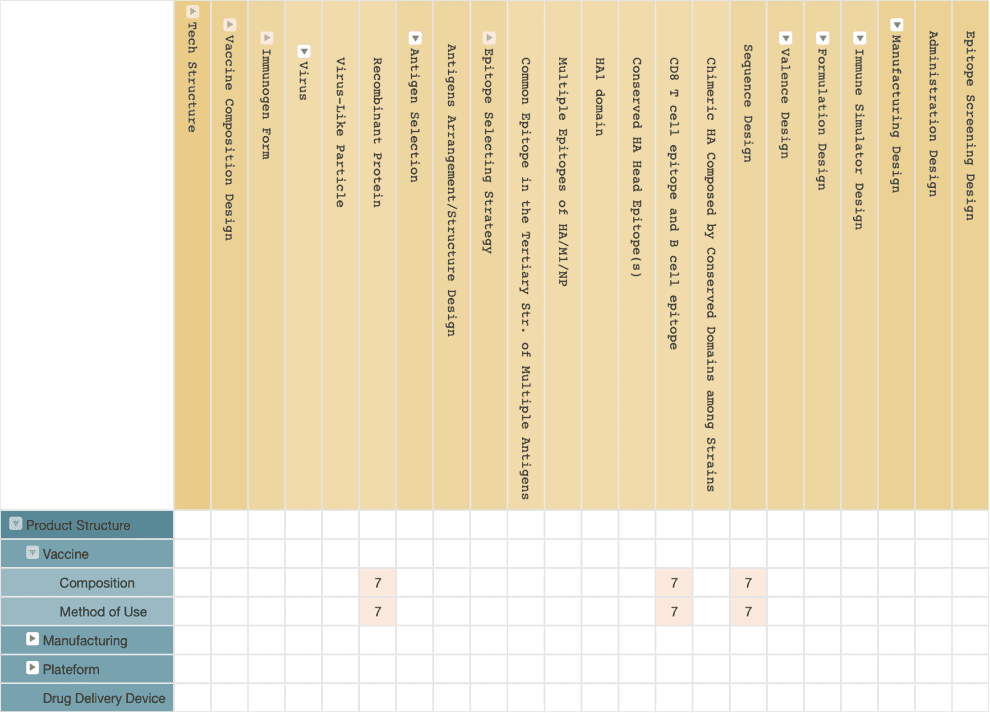
(Source: Wispro Technology Consulting Corporation, 2020)
Distributed Bio
Different from other vaccine development companies, Distributed Bio‘s core competencies lie in its bioinformatic algorithm. The company applies its computational capabilities to a variety of applications, including a universal flu vaccine, a universal snake venom serum, and a human antibody library.
Distributed Bio designed a universal flu vaccine by identifying an HA conserved region which has a 96% identity across 6,500 influenza strains from the tertiary structures of the antigens.
According to the patent application of Distributed Bio, their universal vaccine comprises antigens from at least six influenza strains, where the antigens of these viral strains share a common epitope in tertiary structure.
Through controlling the proportion of various antigens, the concentrations of the individual antigens are not sufficient enough to elicit an immune response, but the sum of all of the common epitopes is enough to elicit an immune response. The company’s initial results for its universal flu vaccine program have been impressive, and it has received $1 million in funding from the Bill & Melinda Gates Foundation and is currently in the preclinical trial phase.
By looking into the company’s patent deployment strategy, we discovered that the company is more focussed on protecting the design concept of multi-antigen vaccines with common epitopes and their epitope screening system rather than protecting the sequence of nucleic acid or amino acid for vaccines, like other vaccine developing companies. In Distributed Bio’s patents, they did not even mention the selected viral antigen in its patent claims.
This patent application approach broadens the patent scope by extending patent claims to cover more general ideas. However, it may encounter difficulties in asserting patent rights since its claims may be hard to judge intuitively during infringement analysis. Besides, those patents related to epitope screening systems could be challenging to prove in an infringement situation, especially in regions other than the United States, since there will be no discovery process in litigation.
Therefore, if Distributed Bio can apply for patents related to a specific antigen sequence at the same time, it can make its patent protection more comprehensive. Since the analysis in this article is based on published patent data, the company may own related patent applications that have not yet been published.
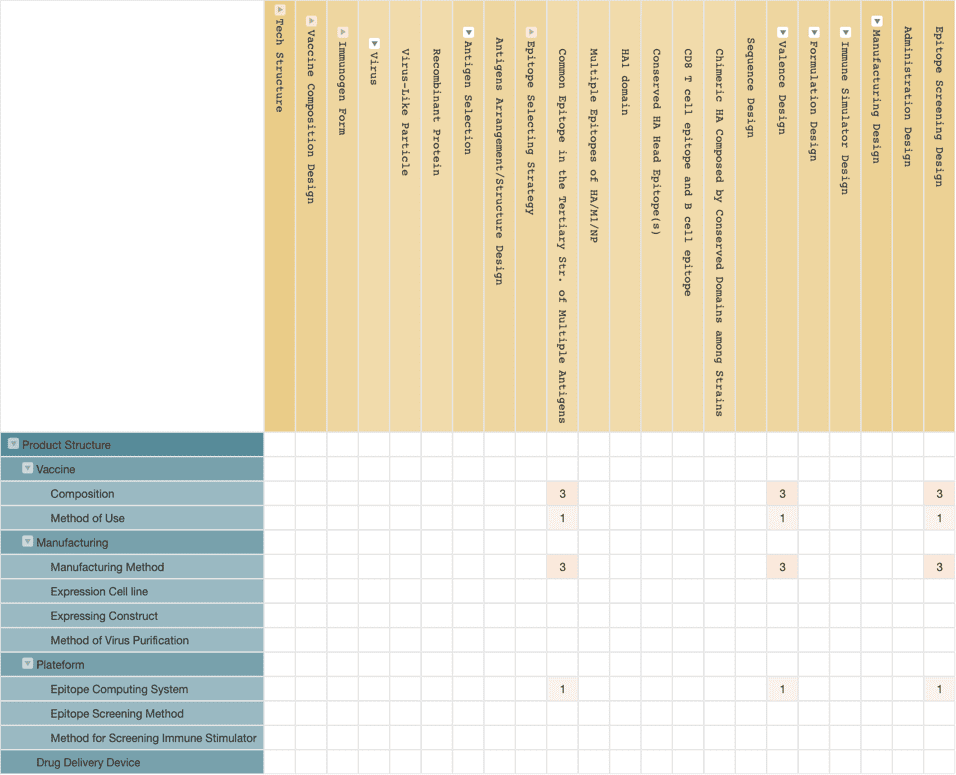
(Source: Wispro Technology Consulting Corporation, 2020)
Compared to recombinant protein subunit vaccines, live attenuated viral vaccines or virus-like particle vaccines have an advantage as these vaccines simulate how the influenza virus actually infects the human body, and can usually produce a more robust immune response. Besides, live attenuated viral vaccines, and virus-like particle vaccines that carry viral surface antigens can often allow vaccination by nasal spray, which is more convenient than injections. Companies developing live attenuated viral vaccines or virus-like particle vaccines include FluGen, Vivaldi Biosciences, Vaccitech, and Blue Water Vaccines.
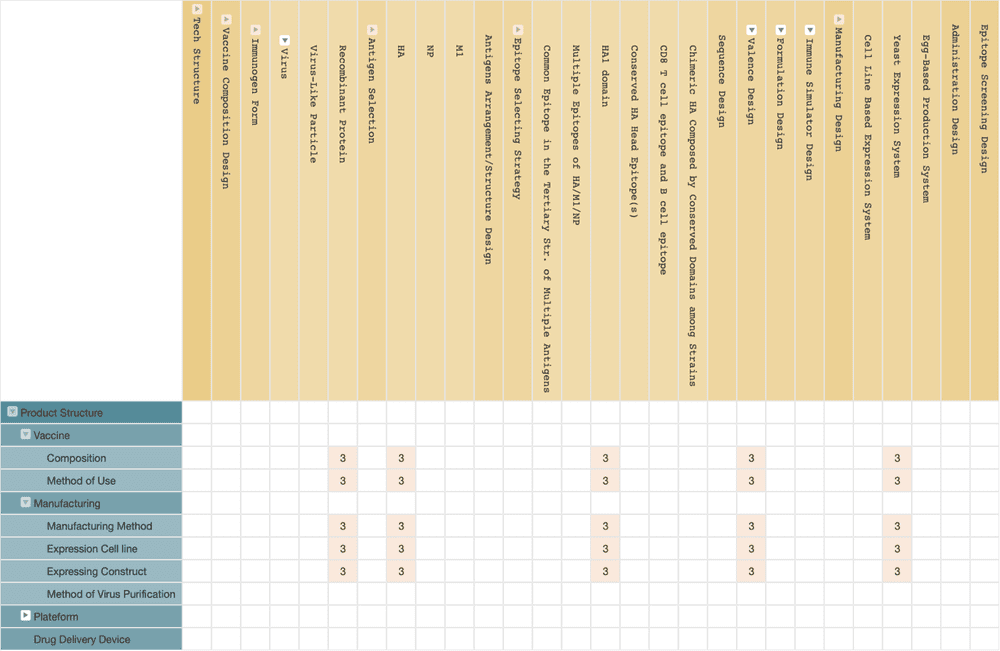
FluGen
The technology of FluGen was developed by Dr. Yoshihiro Kawaoka and Dr. Gabriele Neumann of the University of Wisconsin-Madison. FluGen’s live viral vaccine, M2SR, is based on an influenza virus. Without intact viral Matrix 2 (M2) antigen, the viral vaccine cannot infect human cells. Phase II clinical trials are currently underway.
According to FluGen’s patent portfolio, the company not only applied for viral vaccines with the M2 mutation, but also for a cell line used to produce the virus. Since M2SR is unable to infect human cells without an intact M2 antigen, FluGen uses modified CHO cells with constant influenza M2 gene expression, as a factory for producing M2SR. As the cell line can provide an intact M2 antigen, M2SR would only regain the typical infectivity and replication capabilities in this cell.
Additionally, the cell line can also overexpress the ST6GAL 1 gene, which leads to more 2,6-linked sialic acid production on the cell’s surface. 2,6-linked sialic acids are the key molecules for the influenza virus to enter the cell. Increasing 2,6-linked sialic acids on the host cell’s surface can also increase the efficiency of the virus infectivity to the cell.
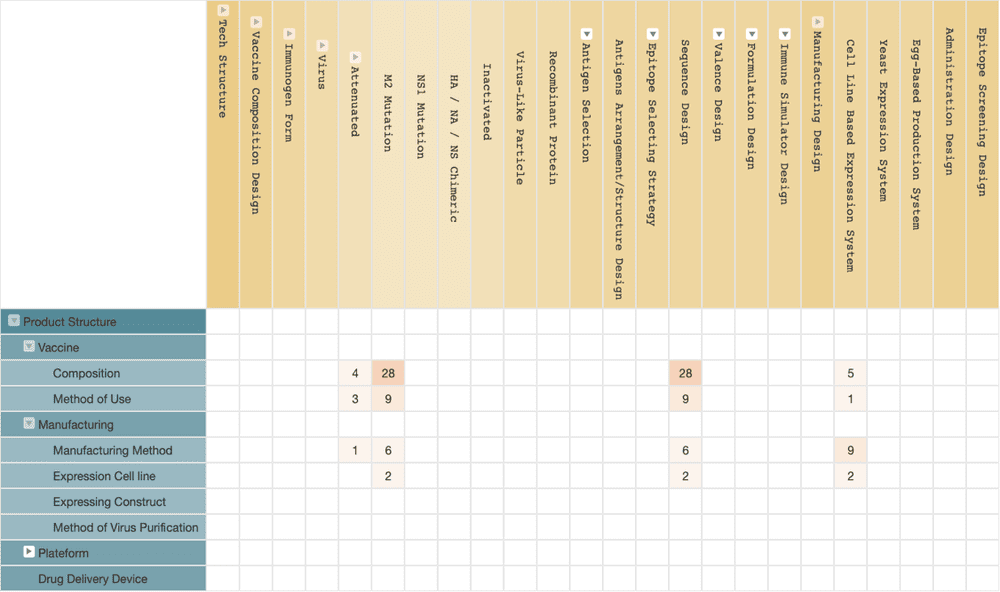
(Source: Wispro Technology Consulting Corporation, 2020)
Vivaldi Biosciences
Vivaldi Biosciences’ live viral vaccine, deltaFLU, is also a genetically deficient influenza virus. Their universal flu vaccine deltaFLU carries the nonstructural protein 1 (NS1) gene mutation. NS1 of the influenza virus has the ability to inhibit the interferon response, which is the crucial immune-stimulating factor we use to fight viral infections. Mutated NS1 in the influenza virus can cause the virus to lose its ability to inhibit interferon, creating a stronger immune response after infection. Therefore, Vivaldi Biosciences’ deltaFLU can provide sufficient immune response without the support of adjuvant.
Although we could not find any patent applications from Vivaldi Biosciences in the public patent data, we did find that two of the company’s founders, Dr. Peter Palese and Dr. Adolfo García-Sastre, and the company’s CSO, Dr. Thomas Muster, had previously worked at the Icahn School of Medicine at Mount Sinai (ISMMS).
When we used each one of them as the inventor to perform a patent search, there were hundreds of patents relating to the NS1 gene mutation influenza virus vaccine, most of which are owned by ISMMS.
Vivaldi Biosciences may have obtained the relevant patent licenses from ISMMS. Vivaldi Biosciences’ technical solutions most likely include the use of chimeric mutations of the HA, Neuraminidase (NA), or NS genes to prevent replication of the influenza virus in human cells.
The chimeric HA gene also provides a chimeric HA antigen that comprises epitopes from more than one strain of the virus, which triggers the immune response to protect against multiple influenza strains.
What’s more, we noticed that BlueSky Vaccines own a small number of related patents. BlueSky Vaccines is a company that focuses on developing virus-based cancer vaccines. According to the company’s press release, we found that BlueSky Vaccines had licensed its technology for virus purification to Vivaldi Biosciences for developing a universal flu vaccine. Interestingly enough, Dr. Thomas Muster, the CEO of BlueSky Vaccines, is also the CSO of Vivaldi Biosciences.
We also noticed that there are some patents related to the production of the NS1 mutant influenza virus, and they are held by Ology Bioservices, a contract development and manufacturing organization (CDMO) specializing in biologic products. This discovery indicates that the two companies may be cooperating with each other. Through patent data analysis, we uncovered the possible relationships between these companies.
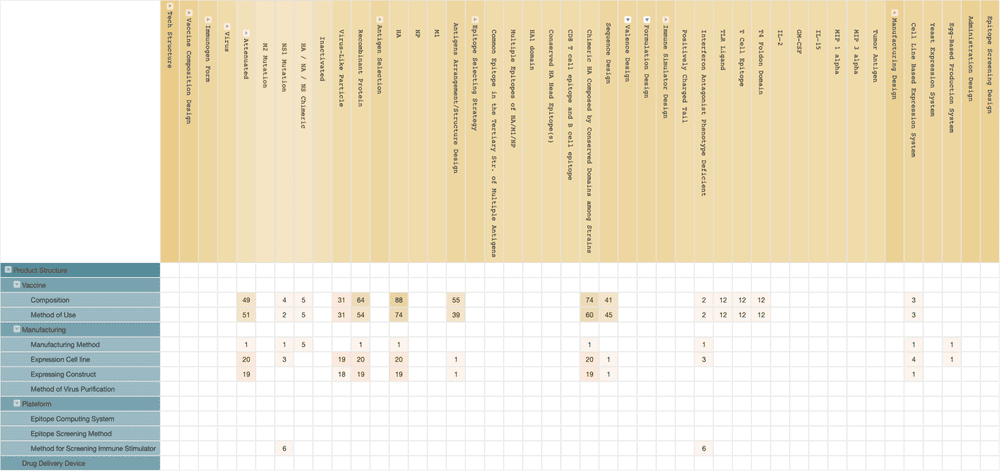
(Source: Wispro Technology Consulting Corporation, 2020)
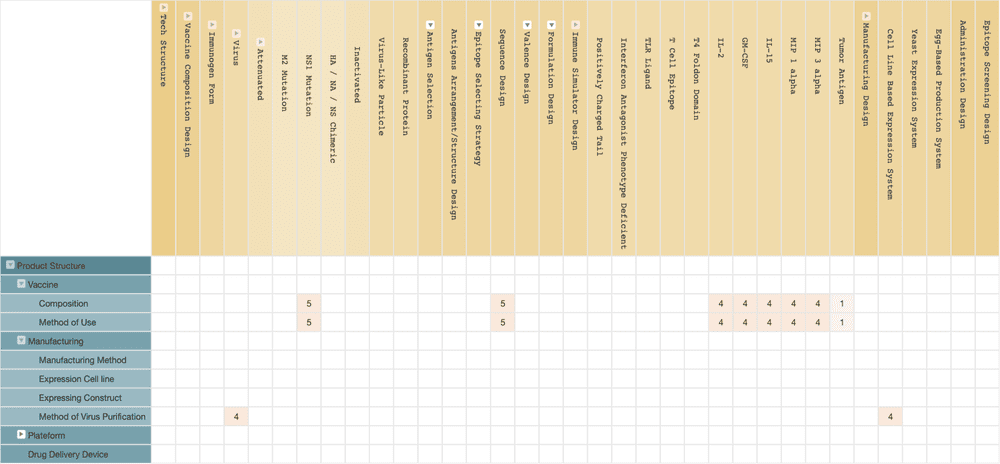
(Source: Wispro Technology Consulting Corporation, 2020)
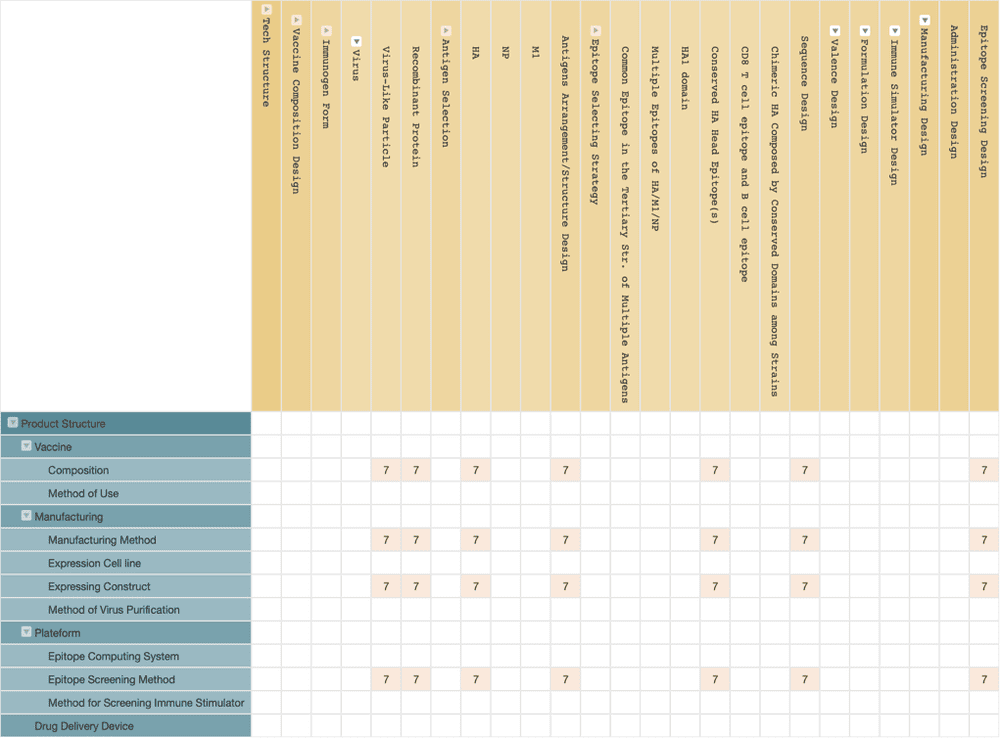
Vaccitech
The universal flu vaccine developed by Vaccitech is a virus-like particle vaccine, which is a vaccine that has a format that is somewhere between a live virus and a recombinant subunit vaccine.
Virus-like particle vaccines do not have the whole structure and nucleic acid of an influenza virus, so they cannot cause illness to humans. However, this kind of vaccine has a virus-like structure and antigens; therefore, they can enter cells in a manner similar to viral infections and may trigger immune responses. Vaccitech’s universal influenza virus VTP-100 is a Modified Vaccinia Ankara (MVA) poxvirus vector with both influenza virus NP and M1 antigens and is currently in phase II of its clinical trial.
The design of VTP-100 is based on a technology developed by the Jenner Institute of Oxford University. According to public patent data, all VTP-100-related patents are currently owned by Oxford University.
Interestingly, we did find patents filed by Vaccitech that relate to an influenza vaccine comprising the HA1 domain subunit and the method for producing it by a yeast expression system. This discovery might be a clue to uncovering their new solution for the universal flu vaccine.
(Source: Wispro Technology Consulting Corporation, 2020)
(Source: Wispro Technology Consulting Corporation, 2020)
Blue Water Vaccines
Blue Water Vaccines is another universal flu vaccine developing company who obtained their technology from Oxford University. There is not much information about what solution the company is adopting. We used the company as the patent assignee to conduct a patent search; however, there aren’t any related patents.
We further searched for patents invented by the company’s CSO, Dr. Craig Thompson, and the technical counsel, Dr. Sunetra Gupta, and we found seven patent applications owned by Oxford University. According to these patents, Blue Water Vaccines’ technology is speculated to be a recombinant protein subunit vaccine or virus-like particle vaccine comprising at least five conserved epitopes in the HA antigen head region of the influenza virus, which were found by algorithms.
Blue Water Vaccines raised approximately $ 7 million in seed funding in 2019 and is expected to start its first clinical trials this year.
The above introduction to universal flu vaccine developing companies shows us how we can use patent data and public information to analyze and survey the technical solutions of specific companies, the development of their technologies, competition, cooperation, relationships, and even the market strategy of each company through analyzing global patent deployment by country.
In fact, patent data analysis plays an essential role in performing due diligence for investment, product research & development, competitor analysis, and finding potential collaborative partners, especially in the technical fields where the product development process is relatively long, such as the fields of biotechnology and pharmaceuticals.
To find out more about our patent data analysis methods, or if you have any comments or ideas related to this article, please feel free to contact us to have a further discussion.
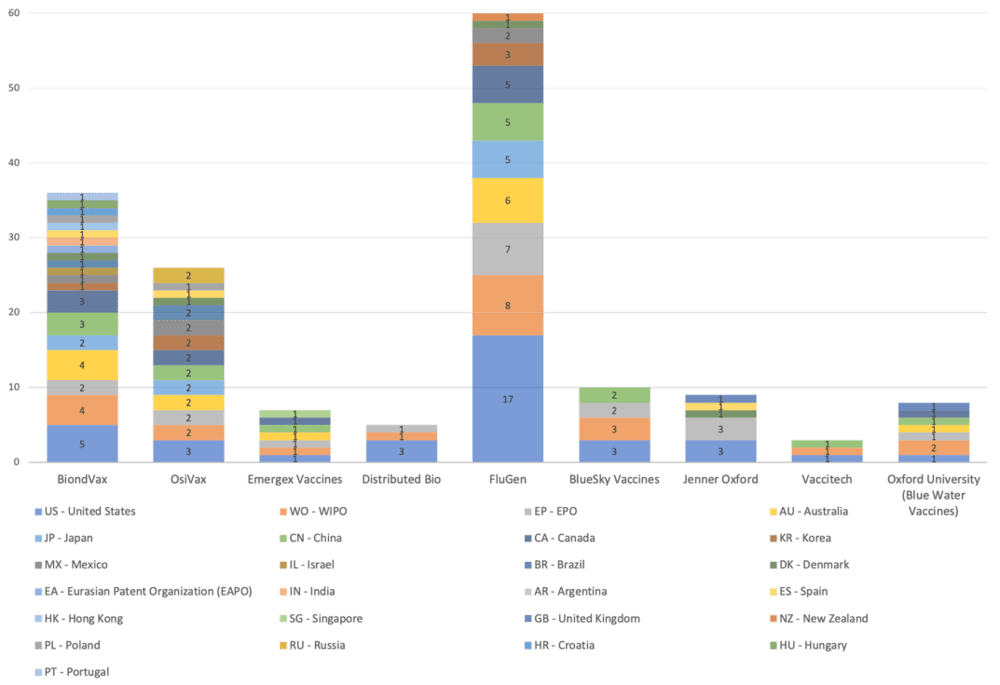
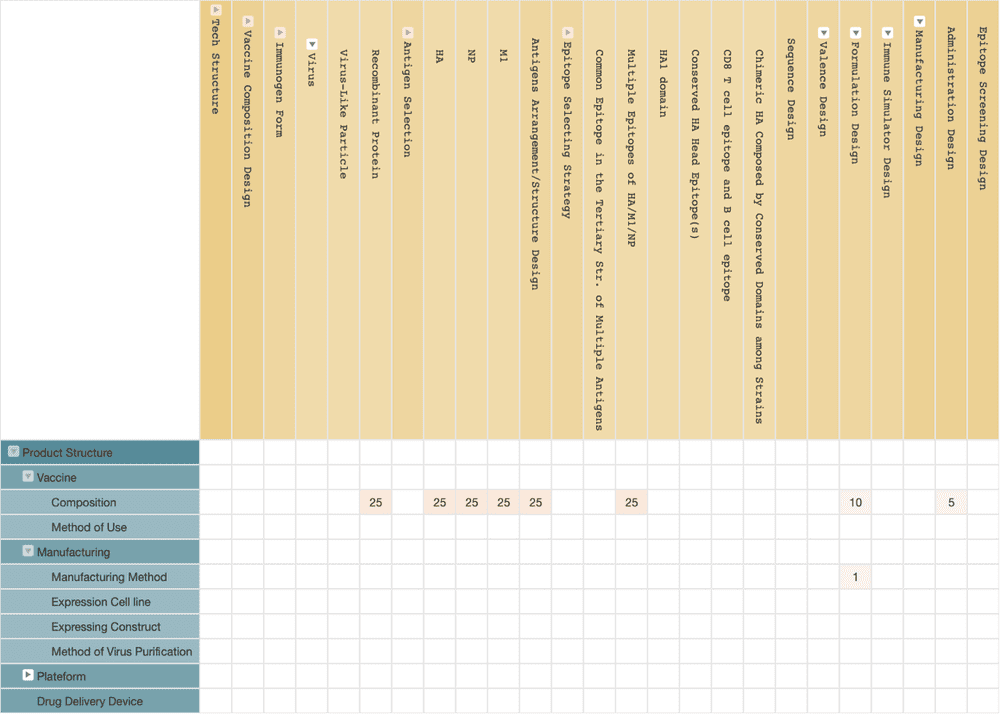
(Source: Wispro Technology Consulting Corporation, 2020)
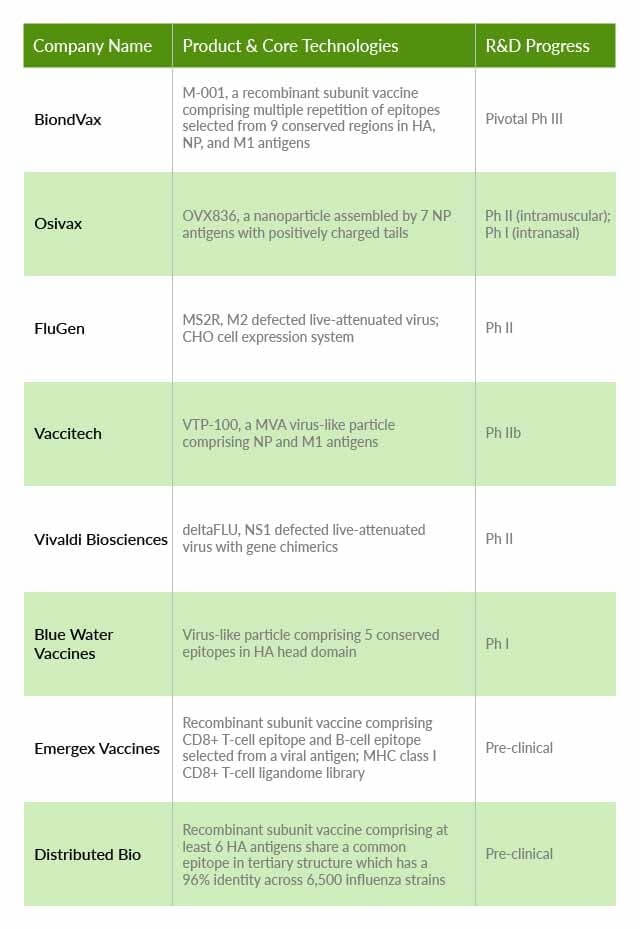
This chart illustrates the vaccine companies mentioned in this article, the products and core technologies they are using, and their various stages of development.
1. Taylor, N. P. (2020, February 11). Trump tries again to reduce NIH budget, spares FDA from cuts. Retrieved March 24, 2020.